Carbon Cycle
This cycle contains any of the natural pathways by which essential elements of living matter are circulated. Biogeochemical cycles are named for the cycling of biological, geological and chemical elements through Earth and its atmosphere.
• The cycles move substances through the biosphere, lithosphere, atmosphere and hydrosphere. Cycles are gaseous and sedimentary.
• Gaseous cycles include nitrogen, oxygen, carbon, phosphorous, sulfur and water.
• These elements cycle through evaporation, absorption by plants and dispersion by wind. Sedimentary cycles include the leeching of minerals and salts from the Earth’s crust, which then settle as sediment or rock before the cycle repeats.
Energy flows through an ecosystem and is dissipated as heat, but chemical elements are recycled.
• For the living components of a major ecosystem (e.g., a lake or a forest) to survive, all the chemical elements that make up living cells must be recycled continuously.
• Energy flows directionally through Earth’s ecosystems, typically entering in the form of sunlight and exiting in the form of heat. However, the chemical components that make up living organisms are different (they get recycled).
• Elements within biogeochemical cycles flow in various forms from the nonliving (a biotic) components of the biosphere to the living (biotic) components and back.
• Repetition of the cycles is important. Plants absorb carbon dioxide and release oxygen, making the air breathable. Plants also acquire nutrients from sediment. Animals acquire nutrients from plants and other animals, and the death of plants and animals returns these nutrients to the sediment as they decay. The cycle then repeats and allows other living things to benefit.
• The simplest example of biogeochemical cycles at work includes water. Water evaporates from the oceans, condenses as clouds and precipitates as rain, which returns the water back to the earth in a cycle.
Many elements cycle through ecosystems, organisms, air, water, and soil. Many of these are trace elements. Other elements, including carbon, nitrogen, oxygen, hydrogen, sulfur, and phosphorus is critical components of all biological life.
Each biogeochemical cycle can be considered as having a reservoir (nutrient) pool a larger, slow-moving, usually abiotic portion and an exchange (cycling) pool a smaller but more-active portion concerned with the rapid exchange between the biotic and abiotic aspects of an ecosystem.
Types of Bio-geochemical Cycles:
From the viewpoint of the ecosphere as a whole, biogeochemical cycles fall into two basic groups:
Gaseous Types, in which the reservoir is in the atmosphere or the hydrosphere (ocean); and
Sedimentary Types, in which the reservoir is in the crust of earth.
CARBON CYCLE
Carbon is a constituent of all organic compounds, many of which are essential to life on Earth. The source of the carbon found in living matter is carbon dioxide (CO2) in the air or dissolved in water. Carbon is found in all organic macromolecules and is also a key component of fossil fuels.
Steps in the carbon cycle
1. Carbon enters the atmosphere as carbon dioxide from respiration and combustion.
2. Carbon dioxide is absorbed by producers to make carbohydrates in photosynthesis.
3. Animals feed on the plant passing the carbon compounds along the food chain. Most of the carbon they consume is exhaled as carbon dioxide formed during respiration. The animals and plants eventually die.
4. The dead organisms are eaten by decomposers and the carbon in their bodies is returned to the atmosphere as carbon dioxide. In some conditions decomposition is blocked. The plant and animal material may then be available as fossil fuel in the future for combustion.
The cycle has four major reservoirs of carbon interconnected by pathways of exchange. The reservoirs are:
1. the atmosphere
2. the terrestrial biosphere (which usually includes freshwater systems and non-living organic material, such as soil carbon)
3. the oceans (which includes dissolved inorganic carbon and living and non-living marine biota
4. the sediments (which includes fossil fuels).
The annual movements of carbon, the carbon exchanges between reservoirs, occur because of various chemical, physical, geological, and biological processes.
Other facts:
- Algae and terrestrial green plants (producers) are the chief agents of carbon dioxide fixation through the process of photosynthesis, through which carbon dioxide and water are converted into simple carbohydrates.
- These compounds are used by the producers to carry on metabolism, the excess being stored as fats and polysaccharides. The stored products are then eaten by consumer organisms, from protozoans to man, which convert them into other forms.
CO2 is added directly to the atmosphere by animals and some other organisms as a by-product of respiration. The carbon present in animal wastes and in the bodies of all organisms is released as CO2 by decay, or decomposer, organisms (chiefly bacteria and fungi) in a series of microbial transformations. - Part of the organic carbon the remains of organisms has accumulated in Earth’s crust as fossil fuels (e.g., coal, gas, and petroleum), limestone, and coral. The carbon of fossil fuels, removed from the cycle in prehistoric time, is now being released in vast amounts as CO2 through industrial and agricultural processes, much of it quickly passing into the oceans and there being “fixed” as carbonates. If oxygen is scarce (as in sewage, marshes, and swamps), some carbon is released as methane gas.
Human Impact on the Carbon Cycle:
- Felling of forests, coal-burning power plants, automobile exhausts, factory smokestacks, and other waste vents of the human environment contribute about 22 billion tons of carbon dioxide (corresponding to 6 billion tons of pure carbon) and other greenhouse gases into the earth’s atmosphere each year. This alters the Carbon Cycle drastically.
- Carbon dioxide emissions are now around 12 times higher than in 1900 because of increased quantities of coal, oil and gas consumption for energy. This serious imbalance in the Carbon cycle is responsible behind the phenomena like Green House Effect, Global Warming and Climate Change. It is now an established fact that this environmental impact will have disastrous consequences for the entire biosphere and humanity.
Oxygen Cycle
Oxygen in the atmosphere is about 21%, and it is the second most abundant gas after nitrogen. It is mostly utilized by living organisms, especially man and animals in respiration. Oxygen is also the most common element of human body.
Oxygen is also used during combustion, decomposition, and oxidation. The circulation of oxygen is through three main flow systems including the (air) atmosphere, the biosphere, and the earth’s crust. In the oxygen cycle, the main driving factor is photosynthesis which is the process whereby green plants and algae make their own food by use of solar energy, water, and carbon dioxide to gives off oxygen as a by-product.
Hence, for oxygen to remain in the atmosphere, it has to circulate through various forms of nature which is essentially termed as the oxygen cycle. The circulation depends on the various activities on Earth.
Oxygen is Produced by:
1. Plants – Plants produce oxygen via photosynthesis
2. Sunlight – Some oxygen is produced when sunlight reacts with water vapour in the atmosphere.
Oxygen is used up in:
• Respiration – All organisms use oxygen for respiration.
• Decomposing – When plants and animals die, they decompose. This process uses up oxygen and releases carbon di oxide into the air.
• Rusting – Also called oxidation, this process causes metals to rust. Also a process which uses up oxygen.
• Combustion – The process by which fire is generated also requires oxygen, along with heat and fuel. This process also uses up oxygen and releases carbon dioxide into the atmosphere.
Other facts
• Plants and animals use oxygen to respire and return it to the air and water as carbon dioxide (CO2).
• CO2 is then taken up by algae and terrestrial green plants and converted into carbohydrates during the process of photosynthesis, oxygen being a by-product.
• The waters of the world are the main oxygen generators of the biosphere; their algae are estimated to replace about 90 percent of all oxygen used.
• Oxygen is involved to some degree in all the other biogeochemical cycles. For example, over time, detritus from living organisms transfers oxygen-containing compounds such as calcium carbonates into the lithosphere.
• Despite the burning of fossil fuel and the reduction of natural vegetation (on land and in the sea), the level of atmospheric oxygen appears to be relatively stable because of the increase in plant productivity resulting from agricultural advances worldwide.
Phosphorus Cycle
- Phosphorus is an essential nutrient for plants and animals in the form of ions PO43– and HPO42–. It is a part of DNA-molecules, of molecules that store energy (ATP and ADP) and of fats of cell membranes. Phosphorus is also a building block of certain parts of the human and animal body, such as the bones and teeth.
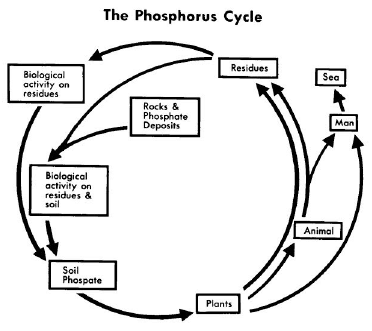
Phosphorus can be found on earth in water, soil and sediments. Unlike the compounds of other matter cycles phosphorus cannot be found in air in the gaseous state. This is because phosphorus is usually liquid at normal temperatures and pressures. It is mainly cycling through water, soil and sediments. - Weathering of rocks and minerals release phosphorus in a soluble form where it is taken up by plants, and it is transformed into organic compounds.
- The plants may then be consumed by herbivores and the phosphorus is either incorporated into their tissues or excreted. After death, the animal or plant decays, and phosphorus is returned to the soil where a large part of the phosphorus is transformed into insoluble compounds. Runoff may carry a small part of the phosphorus back to the ocean.
- In the atmosphere phosphorus can mainly be found as very small dust particles.
Phosphorus moves slowly from deposits on land and in sediments, to living organisms, and then much more slowly back into the soil and water sediment. The phosphorus cycle is the slowest one of the matter cycles. The phosphorus cycle appears somewhat simpler than the nitrogen cycle, because phosphorus occurs in fewer chemical forms.
Parts of the Cycle
- As shown in the Figure, phosphorus, a necessary constituent of protoplasm, tends to circulate with organic compounds in the form of phosphates (PO4), which are again available to plants.
- The great reservoir of phosphorus is not the air, however, but in apatite mineral deposits formed in past geological ages (that is, in the lithosphere). Atmospheric dust and aerosols return a large amount of phosphorus (not phosphate) to the land yearly, but phosphate continually returns to the sea, where part of it is deposited in the shallow sediments and part of it is lost to the deep sediments.
- Contrary to popular belief, seabirds play only a limited role in returning phosphorus to the cycle (as shown by the guano deposits located on the coast of Peru). This transfer of phosphorus and other materials by birds from the sea to the land is continuing, likely at the same rate at which it occurred in the past – but these guano deposits have been mined out.
Human Influence on Phosphorus Cycle
- Human influences on the phosphate cycle come mainly from the introduction and use of commercial synthetic fertilizers. The phosphate is obtained through mining of certain deposits of calcium phosphate called apatite. Huge quantities of sulfuric acid is used in the conversion of the phosphate rock into a fertilizer product called “super phosphate”.
- Plants may not be able to utilize all of the phosphate fertilizer applied, as a consequence, much of it is lost from the land through the water run-off. The phosphate in the water is eventually precipitated as sediments at the bottom of the body of water. In certain lakes and ponds this may be redissolved and recycled as a problem nutrient.
- Animal wastes or manure may also be applied to the land as fertilizer. If misapplied on frozen ground during the winter, much of it may lost as run-off during the spring thaw.
- Other human sources of phosphate are in the form of out flows from municipal sewage treatment plants. Without an expensive tertiary treatment, the phosphate in sewage is not removed during various treatment operations. Again an extra amount of phosphate enters the water. Excess exploitation of the apatite and guano rocks is also making the phosphorus cycle much less cyclic.
