Context
- The Indian Council of Medical Research (ICMR) launched India’s first large-scale trial for two new tuberculosis (TB) vaccines.
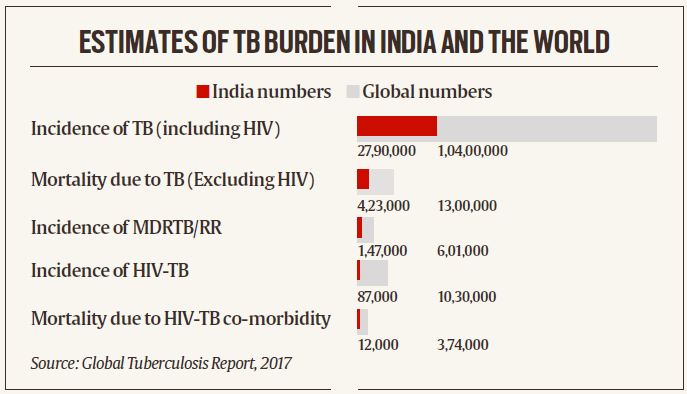
- As per the 2018 annual report of the Central TB division of Ministry of Health and Family Welfare, the incidence of TB was nearly 2.8 million annually, and the incidence of multidrug-resistant TB was 1,47,000 per year.
- The total number of deaths because of TB (excluding HIV) was 4,23,000, and the incidence of HIV-TB was 87,000 per year. India contributes to 27 per cent of the global TB burden; the highest share globally. That is why, in 2017, the central government had committed itself to eliminating TB by 2025.
- The new vaccines that are being put through the trials offer a chance to contain the accelerating spread of multi-drug resistant TB.
- Treating TB requires a multi-drug course of treatment lasting six months; longer still for treating drug-resistant TB.
- Treatment failure and recurrence can have devastating consequences.
Why new vaccines
- Scientists at the Indian Council of Medical Research have felt a critical need for new TB vaccines that are more effective than the Bacille Calmette-Guerin (BCG) vaccine. The BCG vaccine is used in the routine Expanded Programme of Immunisation (EPI) in countries across the world. It is generally given at birth or in the first year. The vaccine is over 100 years old and, while it has been partially effective in protecting infants and young children, particularly from the most severe forms of TB, it provides poor protection against pulmonary disease in adolescents and adults.
Which vaccines
- There are two vaccines being tested in the latest trial: Immuvac (also known as mycobacterium indicus pranii or MIP), which is manufactured by Cadila Pharmaceuticals in Ahmedabad, and VPM1002 manufactured by Serum Institute of India in Pune. There are seven main centres with six subsites where the trial will be conducted.
What the latest trials entail
- Typically, vaccine trials have three phases. During Phase 1, small groups of people receive the trial vaccine. In Phase 2, the clinical study is expanded and the vaccine is given to people who have characteristics (such as age and physical health) similar to those for whom the new vaccine is intended. In Phase 3 the vaccine is given to several thousands of people and tested for efficacy and safety.
- Even so, India’s trials stand out. Dr Manjula Singh, co-investigator and coordinator of the vaccine trial said that nowhere has a Phase 3 trial involved trying out two TB vaccines in one go. This is the first time that such a large preventive TB vaccine trial has been taken up.
- The trials in India will involve enrolling 12,000 healthy household contacts of newly diagnosed pulmonary TB patients. The candidates will be enrolled from across six states — Delhi, Maharashtra, Telangana, Odisha, Karnataka and Tamil Nadu. Approximately 2,000 participants will be enrolled in each state over the next 7-8 months. The main aim of the trial is to evaluate the efficacy of both the vaccines by comparing the reduction in the incidence of TB over a three-year period. The candidates in this trial will be at high risk of contracting the disease, and will be vaccinated in a double-blind manner with either one of the vaccines, and compared with placebo for its efficacy.
What happens next
- This is a Phase 3 vaccine trial where the safety and efficacy of the two TB vaccines are being studied in comparison to the placebo in a larger population.
- Depending on the results, the recommendations would be sent to the Union Ministry of Health and Family Welfare. Both vaccines are being manufactured by Indian pharmaceutical companies. The price of the vaccines would be decided by the government.
Source:IE